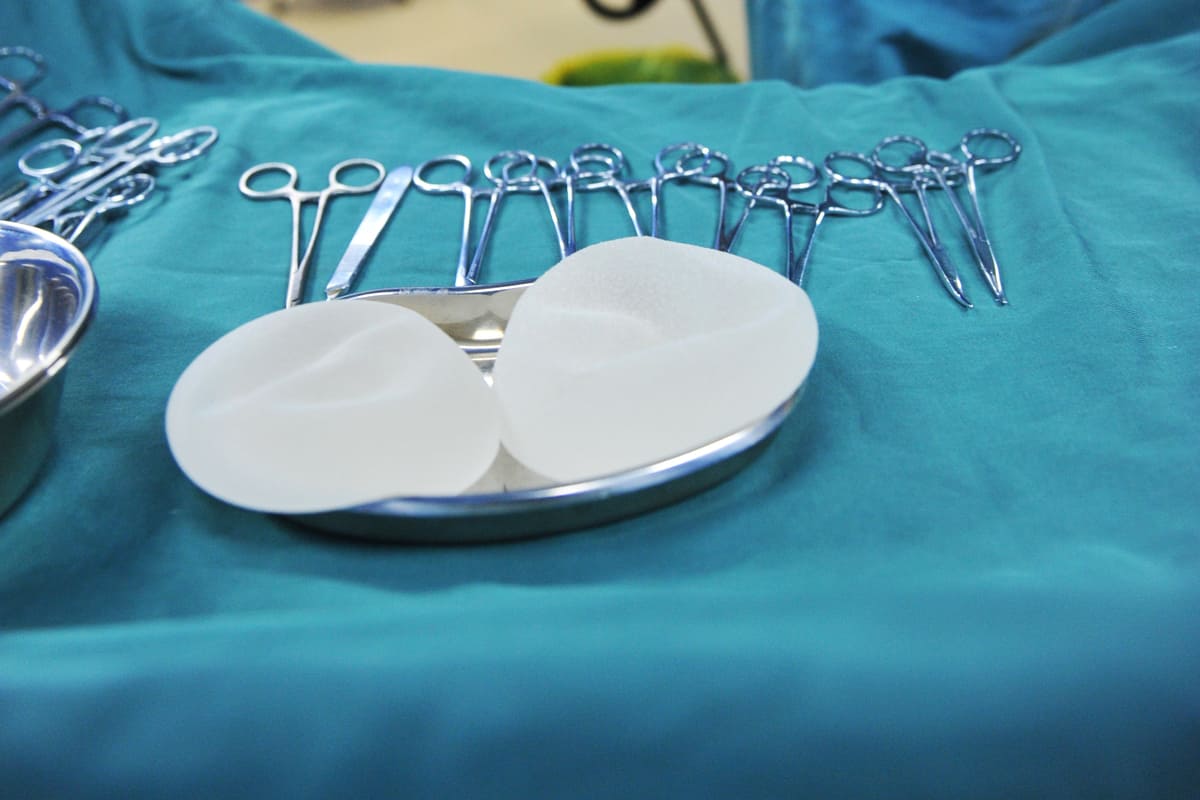
After years of debate, the Food and Drug Administration finally acknowledged what a number of women have known for over three decades. Silicone and saline breast implants cause profound medical issues. Implanted women have endured multiple symptoms for years and some women have died. After reviewing evidence from hundreds of medical device reports, the FDA determined that the implants cause a non-Hodgkin’s Lymphoma known as breast implant-associated anaplastic large cell lymphoma or BIA-ALCL.
On June 24, 2019, the FDA formally requested that Allergan voluntarily recall BIOCELL® textured breast implants and expanders. Allergan complied with the FDA’s request and issued a product safety alert and product recall. The recall covers only the company’s BIOCELL® textured breast implants and tissue expanders. They recalled doctors’ and hospitals’ unused stock and suspended future sales of BIOCELL® branded medical devices. Allergan recommended that women with implanted devices not have them removed unless they were experiencing symptoms.
The FDA is contemplating additional steps to protect future implant patients. The agency began the process by publishing the draft guidance document, “Breast Implants—Certain Labeling Recommendations to Improve Patient Communication.” The FDA wishes to determine the efficacy and necessity of proposed implant packaging and labeling changes. They also want feedback on their recommendation to place a boxed warning on each package.
Women With Implants Have Suffered for Years
At Allan Berger & Associates, we believe that the Allergan recall was an acceptable initial effort but it wasn’t enough. The company has taken no initiative to indemnify women who have spent years suffering due to defective medical devices. We believe that Allergen should pay for the pain and suffering their defective products caused. The attorneys in our Pharmaceutical and Product Device Liability Practice Group are determined to work toward that goal.
The FDA Breast Implant Draft Guidance
The FDA creates draft guidance documents in compliance with the Title 21 Federal Food and Drug Code requirement that they follow Good Guidance Practices. A guidance document explains how the FDA interprets a policy or a regulatory issue. It seeks feedback about concerns such as design, production, labeling, promotion, manufacturing, testing, inspection and enforcement policies.
On October 24, 20129, FDA posted the draft guidance document, “Breast Implants—Certain Labeling Recommendations to Improve Patient Communication,” at Regulation.gov. The FDA sought public input as it’s a key element of the draft guidance process. In this instance the FDA seeks responses concerning several issues:
- Labeling content and format changes
- Updated/additional labeling information: silicone gel-filled breast implant rupture screening recommendations, an easy-to-find description of materials, provision of patient device cards
- Benefits and risks of smooth and textured breast implants
- A proposed box warning and patient decision checklist
Comments on the FDA’s Breast Implant Draft Guidance
Since the FDA posted its breast implant draft guidance, 1,342 people have posted comments. Responses continued after the December 13, 2019 comment closing date until the end of the year. Individuals posted from the UK, France, Lithuania, Canada, and all across the United States. Their remarks vary widely. Some express frustration at finding no medical relief for their conditions. Others discuss years of problems and share long lists of symptoms. Most support several common ideas: implementing a stringent boxed warning, better patient explanations, and the need for more information about possible negative outcomes.
From the content and tone of the posted responses, the implant problem isn’t related to a single implant product or manufacturer. This sampling of comments reveals a variety of adverse experiences.
- October 30, 2019: “I Explanted my Textured Breast Implants last Wednesday. They made me very sick and consumed my life. I have experienced the worst health of my life since having them in… Women should absolutely be warned that these risks and symptoms/ side effects of breast implants are incredibly real and possible…”
- November 1, 2019: “My first sign, as well as several other ladies I know, of breast implant illness, was a ruptured brain aneurysm… These are beyond dangerous they are deadly and no one informed us of this nor did they care…”
- November 13, 2019: “I am an RN and will be an NP in a few months with the goal of acting as a primary care clinician. I am writing to express my concern about the safety of breast implants and to urge the FDA to strengthen the drafted guidance entitled Breast Implants Certain Labeling Recommendations to Improve Patient Communication…”
- December 27, 2019: “I had textured saline implants put in 1998 without so much as a peep of the dangers. I have had things very wrong with me: joints, ringing in ears, discomfort in my chest, and a positive blood test for Scleroderma… I had my implants removed on December 19, 2019.”
- December 27, 2019: “Please consider stricter warnings and for information to be provided to women considering breast implants. I have had implants for 23 years and have been incredibly sick for the past ten years…”
- December 30, 2019: “I was told NOTHING about the possibility for adverse health consequences and risks when I received my Mentor MemoryGel Silicone Implants in June 2007. My health was perfect, zero health conditions… Within 5 years I suffered from numerous medical conditions…”
What Happens Next?
A Food and Drug Administration’s draft guidance doesn’t compel the FDA to commit to any recommendation or policy. It simply explains their position about a pending issue and allows concerned citizens to express their opinions. Once they finalize the guidance, it will provide a summary of the agency’s current thinking about breast implants. This provides an opportunity for the FDA to review the issues and mandate proper labeling to warn future patients of the potential dangers.
The process does not force a negligent manufacturer to pay injured women for the damages they cause. It’s up to each injured person to seek professional legal help.
Allan Berger & Associates Attorney at Law
If a defective breast implant or other medical device injured you or a loved one, our Pharmaceutical and Product Device Liability Practice Group is always here to help. For decades, our law firm has helped our clients recover personal injury damages. We’d like to discuss your case and determine if we can do the same for you. Call us at 504-526-2222 or complete our Contact Form to schedule your free consultation.